What Are the Different Ways to Represent Compounds? Check All That Apply.
iii.3: Representing Compounds- Chemical Formulas and Molecular Models
- Page ID
- 37953
Chemistry is the experimental and theoretical study of materials on their properties at both the macroscopic and microscopic levels. Understanding the human relationship between backdrop and structures/bonding is likewise a hot pursuit. Chemistry is traditionally divided into organic and inorganic chemistry. The onetime is the study of compounds containing at to the lowest degree i carbon-hydrogen bonds. Past default, the chemical study of all other substances is chosen inorganic chemistry, a less well defined subject.
However, the boundary between organic and inorganic compounds is not always well defined. For example, oxalic acid, HtwoC2Ofour, is a compound formed in plants, and information technology is generally considered an organic acid, but it does non contain any C-H bond. Inorganic chemical science is also closely related to other disciplines such as materials sciences, physical chemistry, thermodynamics, world sciences, mineralogy, crystallography, spectroscopy etc.
A chemical formula is a format used to limited the structure of atoms. The formula tells which elements and how many of each element are nowadays in a chemical compound. Formulas are written using the elemental symbol of each atom and a subscript to denote the number of elements. This note can exist accredited to Swedish pharmacist Jons Jakob Berzeliu. The almost common elements present in organic compounds are carbon, hydrogen, oxygen, and nitrogen. With carbon and hydrogen present, other elements, such as phosphorous, sulfur, silicon, and the halogens, may exist in organic compounds. Compounds that do not pertain to this rule are chosen inorganic compounds.
Molecular Geometry and Structural Formula
Understanding how atoms in a molecules are bundled and how they are bonded together is very important in giving the molecule its identity. Isomers are compounds in which two molecules can take the same number of atoms, and thus the same molecular formula, only can have completely different physical and chemical properties considering of differences in structural formula.
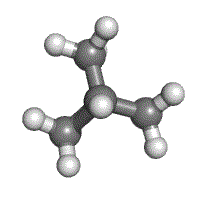

1000 ethylpropane and butane take the same molecular formula of C4Hx, but are structurally unlike (methylpropane on the left, butane on the correct).
Polymers
A polymer is formed when small molecules of identical construction, monomers, combine into a big cluster. The monomers are joined together past covalent bonds. When monomers repeat and bind, they grade a polymer. While they tin can exist comprised of natural or constructed molecules, polymers often include plastics and rubber. When a molecule has more than 1 of these polymers, foursquare parenthesis are used to evidence that all the elements inside the polymer are multiplied by the subscript outside of the parenthesis. The subscript (shown equally n in the example below) denotes the number of monomers present in the macromolecule (or polymer).
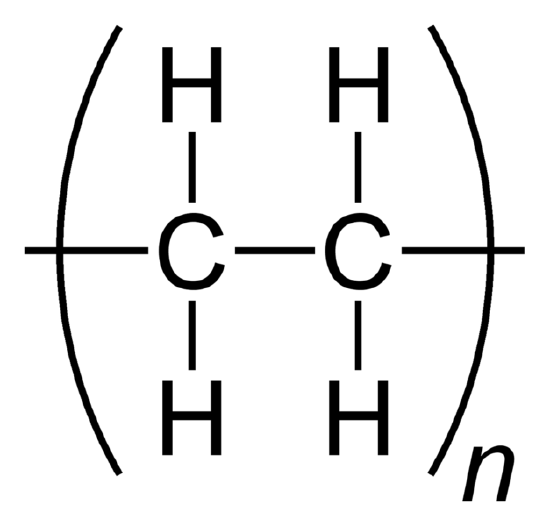
Ethylene becomes the polymer polyethylene.
Molecular Formula
The molecular formula is based on the actual makeup of the compound. Although the molecular formula can sometimes be the same as the empirical formula, molecular compounds tend to exist more than helpful. However, they do non draw how the atoms are put together. Molecular compounds are too misleading when dealing with isomers, which accept the aforementioned number and types of atoms (come across in a higher place in molecular geometry and structural formula).
Ex. Molecular Formula for Ethanol: C2H6O.
Empirical Formula
An empirical formula shows the near basic class of a chemical compound. Empirical formulas testify the number of atoms of each chemical element in a compound in the nearly simplified state using whole numbers. Empirical formulas tend to tell us very little nigh a compound because 1 cannot decide the structure, shape, or properties of the compound without knowing the molecular formula. Usefulness of the empirical formula is decreased because many chemical compounds tin can have the same empirical formula.
Ex. Find the empirical formula for C viii H 16 O 2 .
Answer: CivH eight O (split all subscripts by ii to get the smallest, whole number ratio).
Structural Formula
A structural formula displays the atoms of the molecule in the lodge they are bonded. It likewise depicts how the atoms are bonded to ane another, for example unmarried, double, and triple covalent bond. Covalent bonds are shown using lines. The number of dashes indicate whether the bond is a single, double, or triple covalent bond. Structural formulas are helpful considering they explain the properties and structure of the compound which empirical and molecular formulas cannot e'er stand for.
Ex. Structural Formula for Ethanol: 
Condensed Structural Formula
Condensed structural formulas show the order of atoms like a structural formula only are written in a single line to save space and make it more user-friendly and faster to write out. Condensed structural formulas are also helpful when showing that a group of atoms is connected to a single atom in a compound. When this happens, parenthesis are used effectually the group of atoms to show they are together.
Ex. Condensed Structural Formula for Ethanol: CHiiiCHiiOH (Molecular Formula for Ethanol CtwoHhalf-dozenO).
Line-Angle Formula
Because organic compounds can be complex at times, line-angle formulas are used to write carbon and hydrogen atoms more efficiently past replacing the letters with lines. A carbon atom is present wherever a line intersects another line. Hydrogen atoms are so causeless to complete each of carbon's four bonds. All other atoms that are connected to carbon atoms are written out. Line angle formulas assistance show structure and guild of the atoms in a compound making the advantages and disadvantages like to structural formulas.
Ex. Line-Bending Formula for Ethanol: 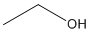
Formulas of Inorganic Compounds
Inorganic compounds are typically not of biological origin. Inorganic compounds are made upwards of atoms connected using ionic bonds. These inorganic compounds can be binary compounds, binary acids, or polyatomic ions.
Binary compounds
Binary compounds are formed betwixt ii elements, either a metal paired with a nonmetal or two nonmetals paired together. When a metal is paired with a nonmetal, they form ionic compounds in which one is a negatively charged ion and the other is positvely charged. The internet charge of the chemical compound must then become neutral. Transition metals accept different charges; therefore, information technology is important to specify what type of ion it is during the naming of the compound. When two nonmetals are paired together, the compound is a molecular compound. When writing out the formula, the element with a positive oxidation state is placed first.
Ex. Ionic Chemical compound: BaBrii(Barium Bromide)
Ex. Molecular Compound: Northward2O4 (Dinitrogen Tetroxide)
Binary acids
Binary acids are binary compounds in which hydrogen bonds with a nonmetal forming an acid. However, there are exceptions such every bit NHiii, which is a base. This is because information technology shows no tendency to produce a H+. Because hydrogen is positively charged, it is placed first when writing out these binary acids.
Ex. HBr (Hydrobromic Acrid)
Polyatomic ions
Polyatomic ions is formed when two or more atoms are connected with covalent bonds. Cations are ions that have are postively charged, while anions are negatively charged ions. The about common polyatomic ions that exists are those of anions. The 2 main polyatomic cations are Ammonium and Mercury (I). Many polyatomic ions are typically paired with metals using ionic bonds to course chemical compounds.
Ex. MnOfour - (Polyatomic ion); NaMnO4 (Chemical Chemical compound)
Oxoacids
Many acids have three different elements to form ternary compounds. When i of those 3 elements is oxygen, the acrid is known as a oxoacid. In other words, oxacids are compounds that incorporate hydrogen, oxgygen, and 1 other element.
Ex. HNO3 (Nitric Acid)
Complex Compounds
Certain compounds tin can announced in multiple forms yet mean the aforementioned matter. A common instance is hydrates: water molecules bail to another compound or element. When this happens, a dot is shown between H2O and the other part of the compound. Because the H2O molecules are embedded within the compound, the chemical compound is not necessarily "wet". When hydrates are heated, the water in the compound evaporates and the compound becomes anhydrous. These compounds tin exist used to attract water such as CoCltwo. When CoCl2 is dry out, CoCl2 is a blue color wherease the hexahydrate (written below) is pinkish in color.
Ex. CoCl2 · six H2O
Formulas of Organic Compounds
Organic compounds contain a combination carbon and hydrogen or carbon and hydrogen with nitrogen and a few other elements, such as phosphorous, sulfur, silicon, and the halogens. About organic compounds are seen in biological origin, as they are constitute in nature.
Hydrocarbons
Hydrocarbons are compounds that consist of only carbon and hydrogen atoms. Hydrocarbons that are bonded together with only single bonds are alkanes. The simplest example is methane (shown below). When hydrocarbons have 1 or more double bonds, they are chosen alkenes. The simplest alkene is Ethene (C2Hiv) which contains a double bond between the 2 carbon atoms.
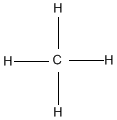
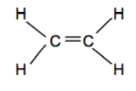
Ex. Methane on left, Ethene on correct
Functional Groups
Functional groups are atoms connected to carbon bondage or rings of organic molecules. Compounds that are inside a functional group tend to accept like properties and characteristics. Ii common functional groups are hydroxyl groups and carboxyl groups. Hydroxyl groups cease in -OH and are alcohols. Carboxyl groups terminate in -COOH, making compounds containing -COOH carboxylic acids. Functional groups likewise help with nomenclature past using prefixes to assist name the compounds that accept similar chemical properties.
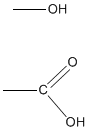
Ex. Hydroxyl Group on peak; Carboxyl Grouping on lesser
References
- Miessler, Gary 50. Inorganic Chemistry. 2nd. Upper Saddle River: Prentince Hall, 1999.
- Munowitz, Michael. Principles of Chemical science. Norton & Company: New York, 2000.
- Pettrucci, Ralph H. General Chemical science: Principles and Mod Applications. ninth. Upper Saddle River: Pearson Prentice Hall, 2007.
Problems
- Which of the following formulas are organic?
- HClO
- C5H10
- CO2
- What is the name of the following formula?
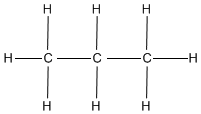
- Classify the following formulas into their appropriate functional group
- Acetic acid
- Butanol
- Oxalic acrid
- What are the empirical formulas for the following compounds?
- C12H10Osix
- CH3CH2CH2CH2CHiiCHiiCH3
- H3O
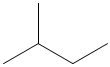
- What is the proper noun of the following figure and what is the molecular formula of the following effigy?
Answer Key:
1. b and c. 2. Propane. 3. a. carboxyl group, b. hydroxyl group, c. carboxyl grouping. 4. a. C6H5O3, b. C7H16, c. HiiiO. 5. Methylbutane, C5H12
Contributors and Attributions
- Jean Kim (UCD), Kristina Bonnett (UCD)
Source: https://chem.libretexts.org/Bookshelves/General_Chemistry/Map:_A_Molecular_Approach_%28Tro%29/03:_Molecules_Compounds_and_Chemical_Equations/3.03:_Representing_Compounds-_Chemical_Formulas_and_Molecular_Models
Post a Comment for "What Are the Different Ways to Represent Compounds? Check All That Apply."